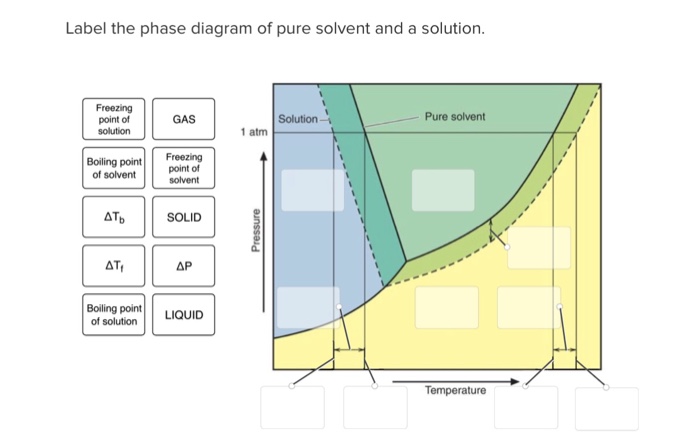
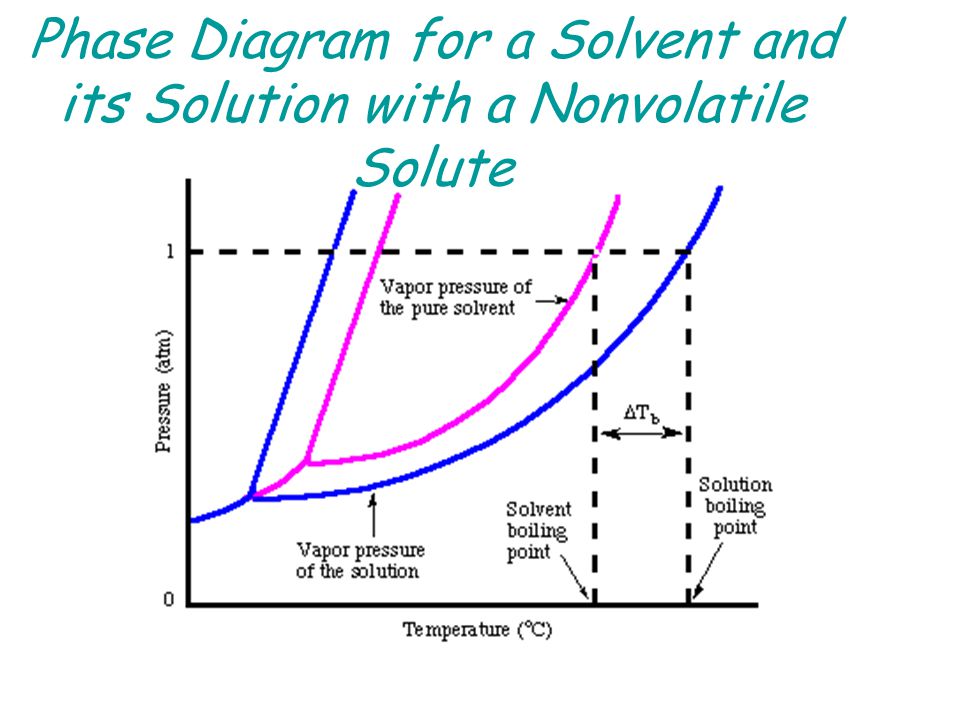
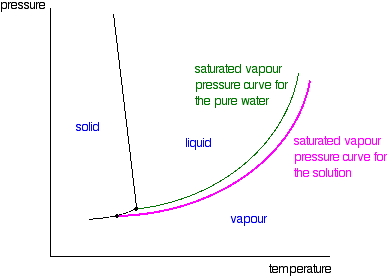
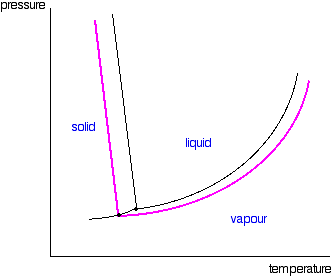
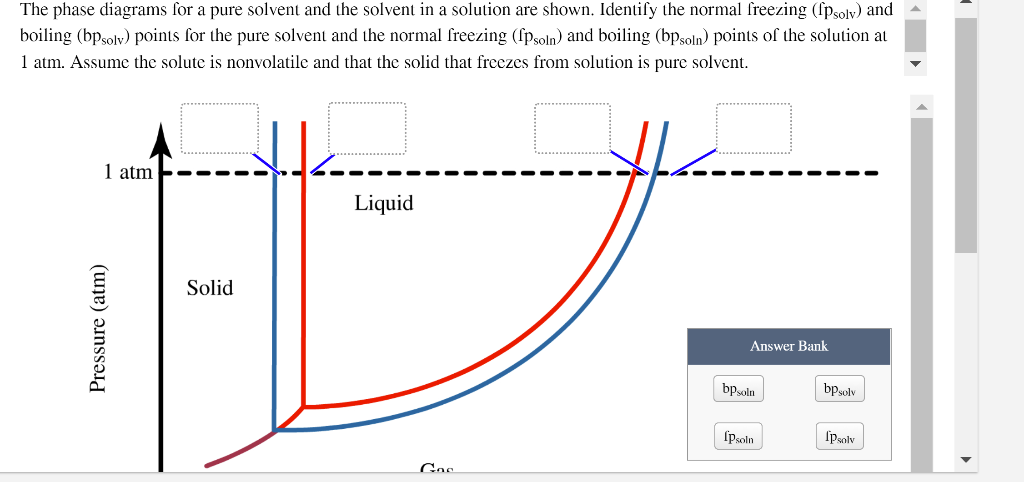
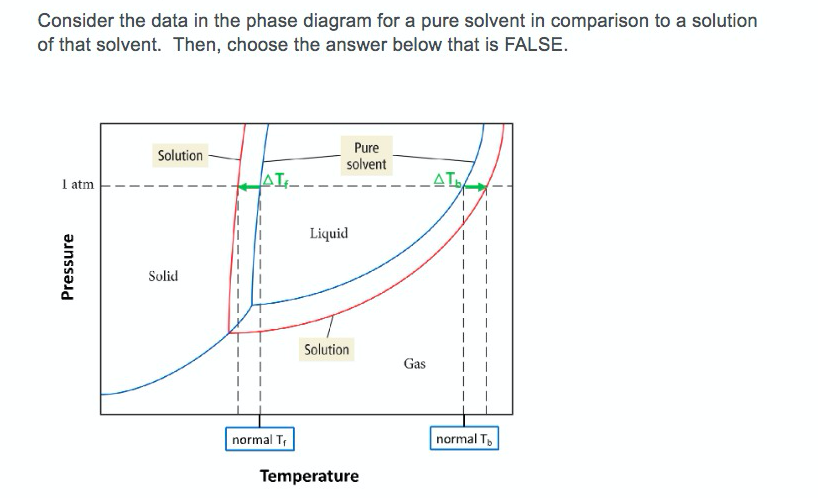
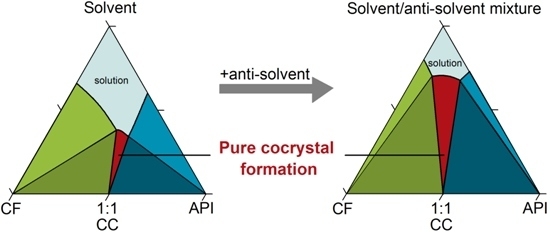
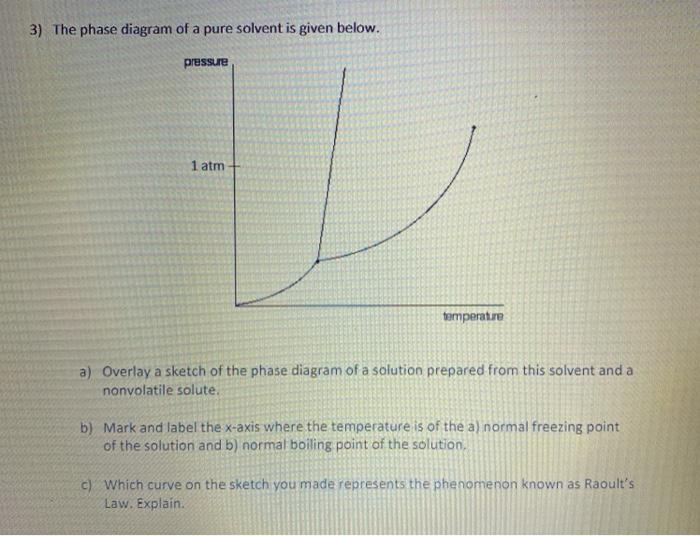
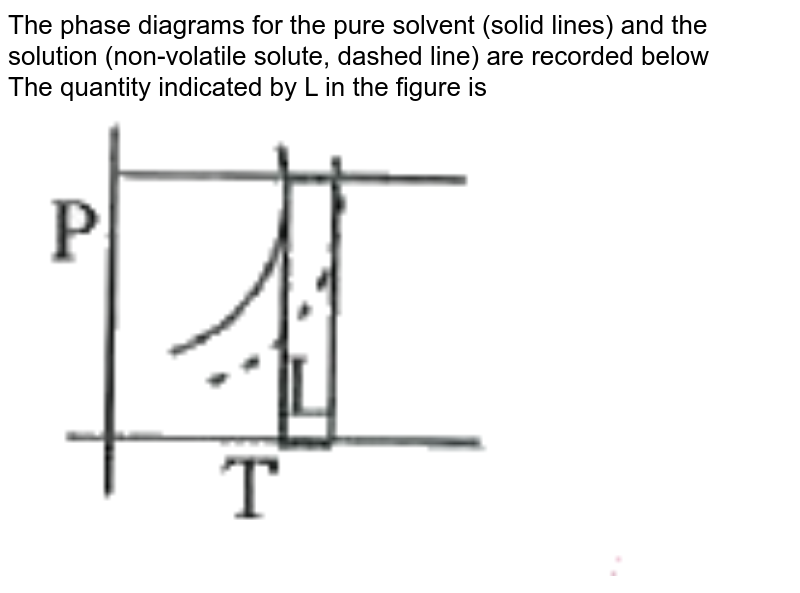
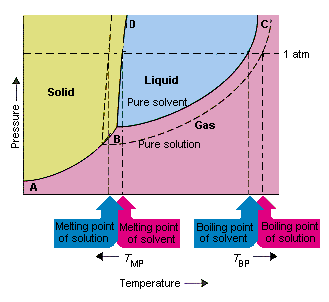
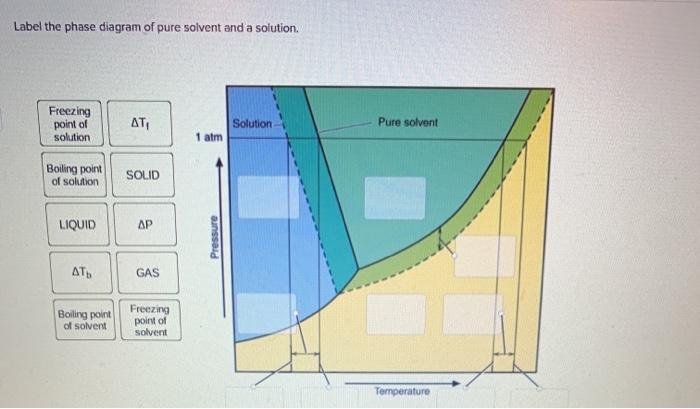
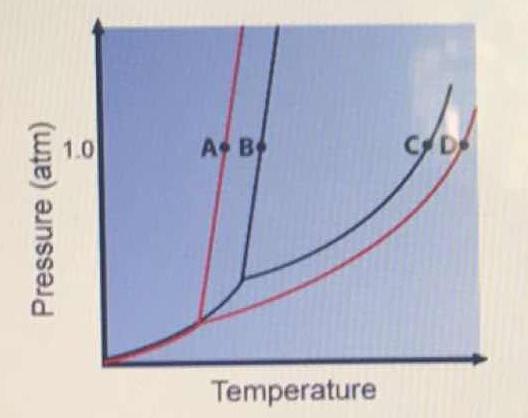
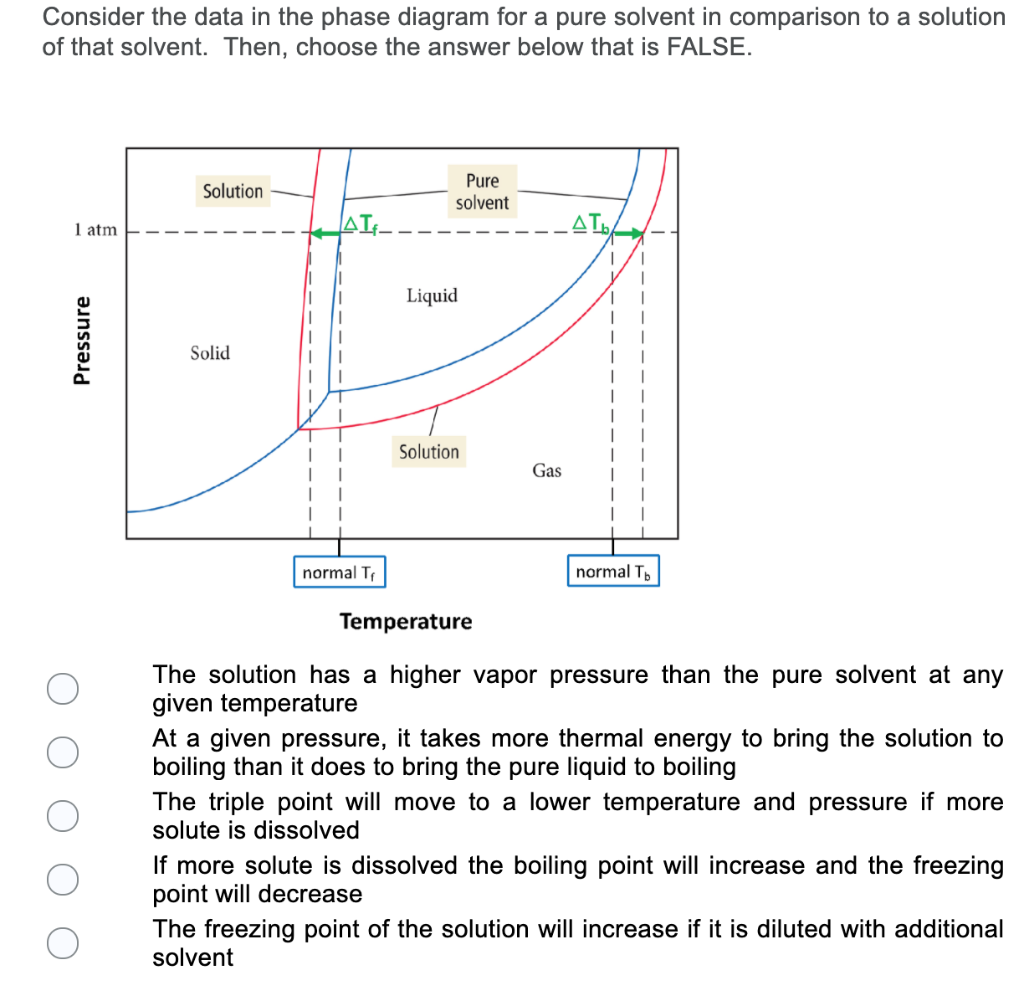
43 phase diagram of pure solvent and solution
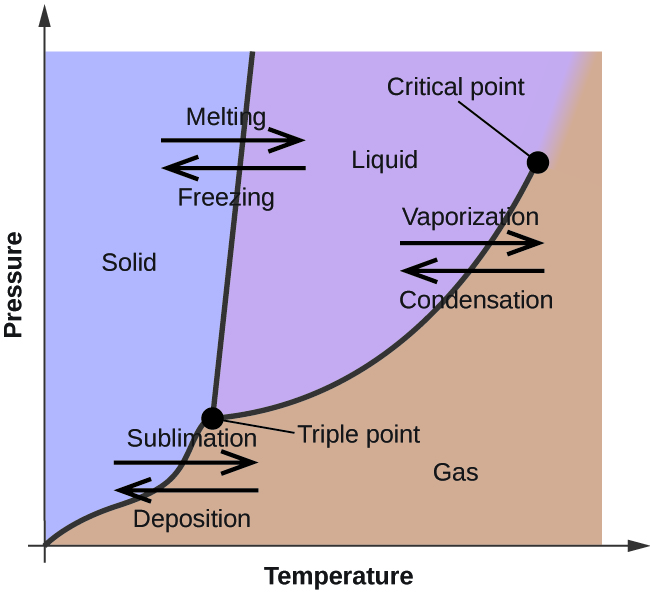
Oxygen - Wikipedia Oxygen is the chemical element with the symbol O and atomic number 8. It is a member of the chalcogen group in the periodic table, a highly reactive nonmetal, and an oxidizing agent that readily forms oxides with most elements as well as with other compounds.Oxygen is Earth's most abundant element, and after hydrogen and helium, it is the third-most abundant element in the … Phase diagram - Wikipedia The simplest phase diagrams are pressure–temperature diagrams of a single simple substance, such as water.The axes correspond to the pressure and temperature.The phase diagram shows, in pressure–temperature space, the lines of equilibrium or phase boundaries between the three phases of solid, liquid, and gas.. The curves on the phase diagram show the points where the …
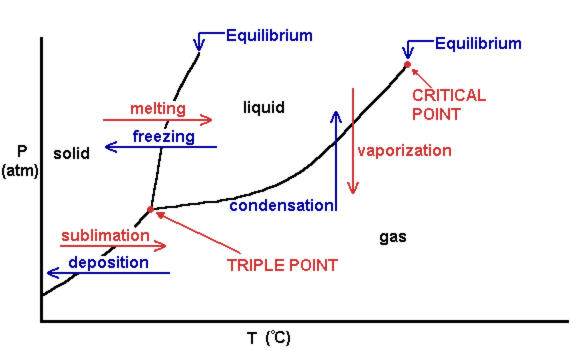
Phase Diagrams – Chemistry - University of Hawaiʻi A phase diagram combines plots of pressure versus temperature for the liquid-gas, solid-liquid, and solid-gas phase-transition equilibria of a substance. These diagrams indicate the physical states that exist under specific conditions of pressure and temperature, and also provide the pressure dependence of the phase-transition temperatures ...

Phase diagram of pure solvent and solution
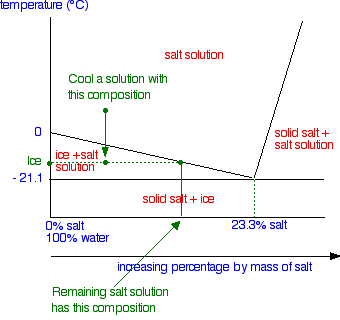
Eutectic system - Wikipedia Sodium chloride and water form a eutectic mixture whose eutectic point is −21.2 °C and 23.3% salt by mass. The eutectic nature of salt and water is exploited when salt is spread on roads to aid snow removal, or mixed with ice to produce low temperatures (for example, in traditional ice cream making).; Ethanol–water has an unusually biased eutectic point, i.e. it is close to pure … Colligative Properties - Purdue University This has no effect on the rate at which solvent molecules in the gas phase condense to form a liquid. But it decreases the rate at which the solvent molecules in the liquid can escape into the gas phase. As a result, the vapor pressure of the solvent escaping from a solution should be smaller than the vapor pressure of the pure solvent. Solid solution - Wikipedia A solid solution, a term popularly used for metals, is a homogenous mixture of two different kinds of atoms in solid state and have a single crystal structure. Many examples can be found in metallurgy, geology, and solid-state chemistry.The word "solution" is used to describe the intimate mixing of components at the atomic level and distinguishes these homogeneous …
Phase diagram of pure solvent and solution. Phase (matter) - Wikipedia Solubility is the maximum amount of a solute that can dissolve in a solvent before the solute ceases to dissolve and remains in a separate phase. ... An unusual feature of the water phase diagram is that the solid–liquid phase line (illustrated by the dotted green line) has a negative slope. ... For pure chemical elements, polymorphism is ... Success Essays - Assisting students with assignments online Each paper writer passes a series of grammar and vocabulary tests before joining our team. Nitrogen - Wikipedia Nitrogen is the chemical element with the symbol N and atomic number 7. Nitrogen is a nonmetal and the lightest member of group 15 of the periodic table, often called the pnictogens. It is a common element in the universe, estimated at seventh in total abundance in the Milky Way and the Solar System.At standard temperature and pressure, two atoms of the element bond to … Mixture - Wikipedia A diagram representing at the microscopic level the differences between homogeneous mixtures, heterogeneous mixtures, compounds, and elements ... a solution has one phase (solid, liquid, or gas), although the phase of the solute and solvent …
Solid solution - Wikipedia A solid solution, a term popularly used for metals, is a homogenous mixture of two different kinds of atoms in solid state and have a single crystal structure. Many examples can be found in metallurgy, geology, and solid-state chemistry.The word "solution" is used to describe the intimate mixing of components at the atomic level and distinguishes these homogeneous … Colligative Properties - Purdue University This has no effect on the rate at which solvent molecules in the gas phase condense to form a liquid. But it decreases the rate at which the solvent molecules in the liquid can escape into the gas phase. As a result, the vapor pressure of the solvent escaping from a solution should be smaller than the vapor pressure of the pure solvent. Eutectic system - Wikipedia Sodium chloride and water form a eutectic mixture whose eutectic point is −21.2 °C and 23.3% salt by mass. The eutectic nature of salt and water is exploited when salt is spread on roads to aid snow removal, or mixed with ice to produce low temperatures (for example, in traditional ice cream making).; Ethanol–water has an unusually biased eutectic point, i.e. it is close to pure …



















Post a Comment for "43 phase diagram of pure solvent and solution"